The Celler Kefauver Act Definition
adminse
Mar 25, 2025 · 9 min read
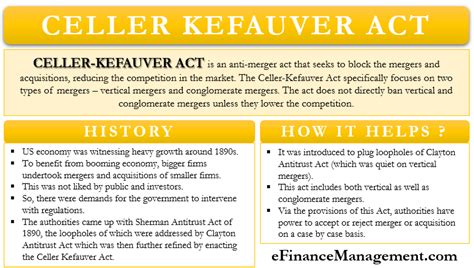
Table of Contents
Unlocking the Power of Drug Safety: A Deep Dive into the Kefauver-Harris Amendment
What if the safety and efficacy of medications were largely unregulated, leaving consumers vulnerable to potentially harmful drugs? The Kefauver-Harris Amendment fundamentally changed the landscape of drug regulation, ushering in an era of increased safety and accountability for pharmaceutical companies.
Editor’s Note: This article on the Kefauver-Harris Amendment (often referred to as the Kefauver-Harris Act) provides a comprehensive overview of this landmark legislation, its historical context, and its lasting impact on the pharmaceutical industry and public health. This updated analysis examines its ongoing relevance and the continuing evolution of drug regulation in its wake.
Why the Kefauver-Harris Amendment Matters:
The Kefauver-Harris Amendment of 1962 represents a pivotal moment in the history of pharmaceutical regulation in the United States. Prior to its enactment, the Food, Drug, and Cosmetic Act of 1938, while a significant improvement over previous legislation, lacked crucial provisions for ensuring drug safety and efficacy. This gap led to several high-profile instances of drug-related harm, most notably the thalidomide tragedy, which tragically resulted in thousands of babies born with severe birth defects. The amendment, named after its sponsors, Senator Estes Kefauver and Representative Oren Harris, addressed these shortcomings by imposing stricter requirements for drug approval, significantly altering the relationship between the pharmaceutical industry and the Food and Drug Administration (FDA). Its implications extend far beyond the initial response to thalidomide, impacting every aspect of drug development, marketing, and regulation. Understanding this act is crucial for anyone interested in pharmaceutical policy, public health, and the complex interplay between science, regulation, and consumer protection.
Overview: What This Article Covers:
This article will explore the historical context that led to the Kefauver-Harris Amendment, defining its key provisions and analyzing its impact on drug development, clinical trials, and marketing practices. We'll examine the long-term consequences of the legislation, including its contribution to increased drug safety, the evolving regulatory landscape, and the ongoing debate surrounding its efficacy and potential limitations. Finally, we will consider the amendment’s lasting legacy and its continuing relevance in the face of contemporary challenges in pharmaceutical regulation.
The Research and Effort Behind the Insights:
This analysis draws upon extensive research, including primary source documents such as the text of the amendment itself, historical accounts of its passage, subsequent FDA regulations, academic literature on pharmaceutical regulation, and analyses of its impact on drug development and public health. The information presented is intended to provide a neutral and authoritative overview of the Kefauver-Harris Amendment and its significance.
Key Takeaways:
- Enhanced Drug Approval Process: The amendment significantly strengthened the drug approval process, mandating rigorous testing of drug safety and efficacy before marketing.
- Increased FDA Authority: The FDA gained substantially more power to regulate the pharmaceutical industry, including the authority to conduct inspections and enforce regulations more effectively.
- Emphasis on Clinical Trials: The amendment formalized the requirements for clinical trials, ensuring that drugs undergo thorough testing before being approved for public use.
- Stricter Advertising Regulations: The amendment introduced stricter regulations on drug advertising, prohibiting false or misleading claims about drug efficacy.
- Good Manufacturing Practices (GMP): The legislation laid the groundwork for the implementation of Good Manufacturing Practices (GMP), ensuring that drugs are manufactured under controlled conditions to maintain consistent quality and purity.
Smooth Transition to the Core Discussion:
Having established the importance and scope of the Kefauver-Harris Amendment, let's delve into its historical context and the specific provisions that transformed the landscape of drug regulation.
Exploring the Key Aspects of the Kefauver-Harris Amendment:
1. Historical Context and the Thalidomide Tragedy:
The Kefauver-Harris Amendment was a direct response to the thalidomide tragedy. Thalidomide, a sedative marketed in Europe and other countries, was found to cause severe birth defects in thousands of children. The drug’s approval in Europe was based on insufficient testing, highlighting significant gaps in existing drug safety regulations. The United States, under the leadership of Dr. Frances Kelsey at the FDA, prevented the widespread marketing of thalidomide, demonstrating the potential for stringent regulation to safeguard public health. This near-miss, however, served as a powerful catalyst for comprehensive legislative reform. The public outcry and Congressional investigations following the thalidomide tragedy galvanized support for stricter regulations, paving the way for the Kefauver-Harris Amendment.
2. Key Provisions of the Amendment:
The Kefauver-Harris Amendment introduced several critical changes to the existing drug approval process:
- Proof of Efficacy: The amendment required pharmaceutical companies to demonstrate not only the safety but also the efficacy of their drugs before marketing. This marked a significant shift from the previous system, where safety was the primary concern.
- Expanded FDA Authority: The FDA gained increased authority to regulate the pharmaceutical industry, including the power to conduct inspections of manufacturing facilities, require the submission of detailed pre-market data, and enforce stricter advertising standards.
- Regulation of Clinical Trials: The amendment formalized the requirements for clinical trials, specifying the need for informed consent from participants, the establishment of ethical review boards (IRBs), and the submission of detailed clinical trial data to the FDA.
- Drug Advertising Regulations: The amendment strengthened advertising regulations, requiring that drug advertisements be truthful, not misleading, and provide complete information about the drug's benefits and risks. This was a direct response to the practice of promoting drugs with exaggerated or unsubstantiated claims.
- Good Manufacturing Practices (GMP): The amendment established Good Manufacturing Practices (GMP) regulations to ensure the consistent quality and purity of manufactured drugs. These regulations cover aspects of manufacturing, quality control, and testing procedures.
3. Impact on Drug Development and Approval:
The Kefauver-Harris Amendment fundamentally altered the drug development process. The requirement for demonstrating both safety and efficacy led to a significant increase in the time and cost associated with bringing new drugs to market. This, in turn, led to a more rigorous approach to drug development, involving extensive preclinical and clinical testing to meet the FDA's stringent approval criteria.
4. Long-Term Consequences:
The Kefauver-Harris Amendment had a profound and lasting impact on the pharmaceutical industry and public health. It led to a significant reduction in the number of drugs causing serious adverse effects. It raised the bar for drug safety and efficacy, improving the overall quality of medications available to consumers. The amendment also resulted in increased transparency and accountability within the pharmaceutical industry, holding companies more responsible for the drugs they produce and market.
However, the amendment's impact was not without its criticisms. The increased regulatory burden resulted in longer approval times and higher costs for developing new drugs. This, critics argue, may have hindered innovation and potentially limited access to some life-saving medications.
Exploring the Connection Between Informed Consent and the Kefauver-Harris Act:
The concept of informed consent is inextricably linked to the Kefauver-Harris Amendment. The amendment's emphasis on rigorous clinical trials made informed consent a central tenet of drug development. Before the amendment, participation in clinical trials was often poorly explained, and individuals were not fully aware of the potential risks.
Key Factors to Consider:
- Roles and Real-World Examples: The Kefauver-Harris Amendment established the ethical framework for informed consent, requiring researchers to fully inform potential participants about the study's purpose, procedures, risks, and benefits. Numerous examples exist of clinical trials conducted since the amendment's passage that adhere to this standard. However, instances of inadequate informed consent also highlight the ongoing need for vigilant oversight.
- Risks and Mitigations: Ensuring truly informed consent can be challenging. Issues such as language barriers, cultural differences, and the inherent complexity of medical information can compromise the process. Mitigating these risks requires carefully designed informed consent processes, translated materials, and culturally competent researchers.
- Impact and Implications: The emphasis on informed consent has fundamentally altered the relationship between researchers and participants, fostering greater trust and respect for individual autonomy. It has also had a broader impact on biomedical research ethics, setting a precedent for informed consent in other areas of medical research.
Conclusion: Reinforcing the Connection:
The emphasis on informed consent, central to the Kefauver-Harris Amendment, underscores the legislation's commitment to protecting individuals while furthering medical progress. By promoting transparency and ethical conduct in clinical trials, the amendment helped establish a foundation for responsible drug development and improved public health.
Further Analysis: Examining Post-Market Surveillance in Greater Detail:
While the Kefauver-Harris Amendment significantly improved pre-market drug safety, it also highlighted the need for ongoing post-market surveillance. Post-market surveillance involves monitoring the safety and efficacy of drugs after they have been approved and marketed. This is crucial because many adverse events may not be detected during pre-market clinical trials.
FAQ Section: Answering Common Questions About the Kefauver-Harris Amendment:
- What is the Kefauver-Harris Amendment? The Kefauver-Harris Amendment, also known as the Drug Amendments of 1962, is a landmark piece of legislation that significantly strengthened the regulation of drugs in the United States.
- What event prompted the amendment? The thalidomide tragedy, which resulted in thousands of children born with severe birth defects, served as the primary catalyst for the amendment's passage.
- What are the main provisions of the amendment? The amendment requires proof of both safety and efficacy before drug approval, grants the FDA greater authority over drug development and marketing, mandates rigorous clinical trials with informed consent, and strengthens advertising regulations.
- What is the impact of the amendment? The amendment led to a significant improvement in drug safety, increased FDA authority, and fundamentally reshaped the drug development and approval process.
Practical Tips: Understanding and Applying the Legacy of the Kefauver-Harris Amendment:
- Stay Informed: Keep abreast of current FDA regulations and updates to pharmaceutical policies.
- Critical Evaluation: Develop skills to critically evaluate drug information, recognizing potential biases in advertising and marketing.
- Advocate for Safety: Support organizations dedicated to promoting drug safety and consumer protection.
Final Conclusion: Wrapping Up with Lasting Insights:
The Kefauver-Harris Amendment stands as a testament to the importance of rigorous drug regulation in safeguarding public health. While it has been refined and expanded upon over the years, its fundamental principles remain critical today, underscoring the need for continuous vigilance in ensuring the safety and efficacy of medications. The amendment's legacy is one of improved consumer protection, enhanced scientific rigor in drug development, and a strengthened partnership between regulators and the pharmaceutical industry—a partnership essential for maintaining public trust and fostering innovation while prioritizing patient safety.
Latest Posts
Related Post
Thank you for visiting our website which covers about The Celler Kefauver Act Definition . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.